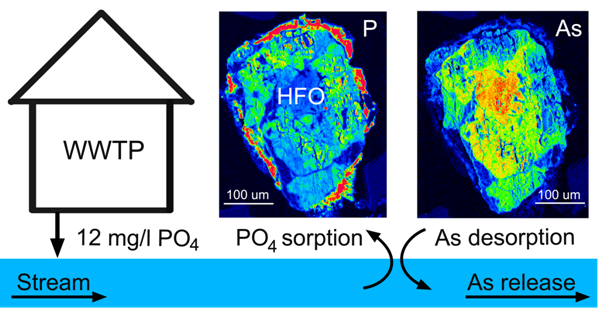
Wastewater treatment plants are an important source of phosphorus to the environment. They are estimated to bring between 25 and 45 % of total phosphorus in freshwaters. This study describes how discharged phosphate releases arsenic from streambed sediments into freshwaters. After seven years, the concentration of total As in the streambed sediment below the wastewater treatment plant decreased by 25 % due competitive desorption of arsenate by phosphate.
Petra Venhauerova, Petr Drahota, Ladislav Strnad, Šárka Matoušková (2022): Effects of a point source of phosphorus on the arsenic mobility and transport in a small fluvial system. Environmental Pollution 315, 120477. (DOI)

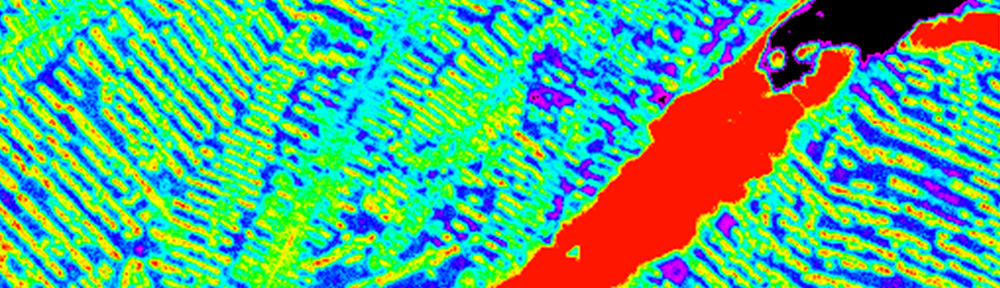



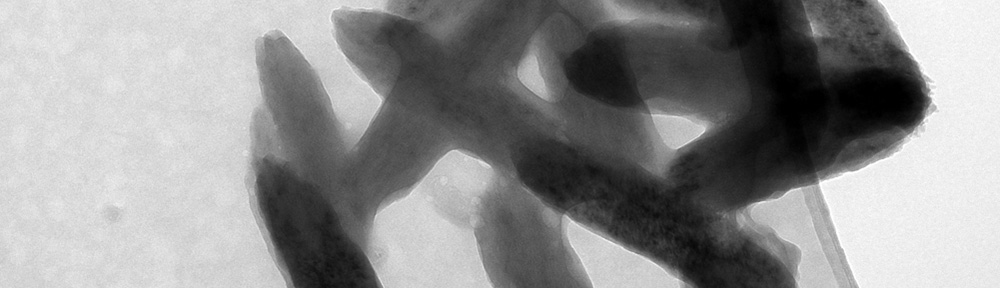

 This study explores the oxidation of arsenopyrite (FeAsS) and löllingite (FeAs2) at high relative humidity (RH: 75%-100%). Long-term oxidation (40 months) experiments show that oxidation of arsenopyrite and löllingite led to formation of different assemblages of secondary phases. Arsenopyrite oxidized to poorly-crystalline ferric arsenate, while löllingite oxidized to scorodite (FeAsO4·2H2O) and arsenolite (As2O3). Our results showed that the exposure of arsenopyrite and löllingite to different RH levels significantly influenced the amounts of newly formed phases. The major environmental impact of sulfide weathering occurs in well aerated environments characterized by high humidity – such as underground workings and some unsaturated mine wastes and tailings deposits.
This study explores the oxidation of arsenopyrite (FeAsS) and löllingite (FeAs2) at high relative humidity (RH: 75%-100%). Long-term oxidation (40 months) experiments show that oxidation of arsenopyrite and löllingite led to formation of different assemblages of secondary phases. Arsenopyrite oxidized to poorly-crystalline ferric arsenate, while löllingite oxidized to scorodite (FeAsO4·2H2O) and arsenolite (As2O3). Our results showed that the exposure of arsenopyrite and löllingite to different RH levels significantly influenced the amounts of newly formed phases. The major environmental impact of sulfide weathering occurs in well aerated environments characterized by high humidity – such as underground workings and some unsaturated mine wastes and tailings deposits.
 We used an in situ experimental technique with double nylon experimental bags to study the effect of low-cost organic materials (sawdust, wood cubes and hemp shives) on As sulfidation in three naturally As-enriched wetland soils. After 15 months of in situ incubation, all of the organic materials were covered by yellow-black mineral accumulations, dominantly composed of crystalline As4S4 polymorphs (realgar and bonazziite) and reactive Fe(II) sulfides (probably mackinawite). Our findings suggest an authigenic formation of AsS minerals in strongly reducing conditions of experimental bags by a combination of reduced exchange of solutes through the pores of the bag and comparatively fast microbial production of dissolved sulfide. Arsenic sulfide formation, as an effective treatment mechanism for natural and human-constructed wetlands, appears to be favored for As(III)-rich waters with a low Fe(II)/As(III) molar ratio, preventing the consumption of dissolved As and sulfide by their preferential incorporation into natural organic matter, and newly-formed Fe(II) sulfides, respectively.
We used an in situ experimental technique with double nylon experimental bags to study the effect of low-cost organic materials (sawdust, wood cubes and hemp shives) on As sulfidation in three naturally As-enriched wetland soils. After 15 months of in situ incubation, all of the organic materials were covered by yellow-black mineral accumulations, dominantly composed of crystalline As4S4 polymorphs (realgar and bonazziite) and reactive Fe(II) sulfides (probably mackinawite). Our findings suggest an authigenic formation of AsS minerals in strongly reducing conditions of experimental bags by a combination of reduced exchange of solutes through the pores of the bag and comparatively fast microbial production of dissolved sulfide. Arsenic sulfide formation, as an effective treatment mechanism for natural and human-constructed wetlands, appears to be favored for As(III)-rich waters with a low Fe(II)/As(III) molar ratio, preventing the consumption of dissolved As and sulfide by their preferential incorporation into natural organic matter, and newly-formed Fe(II) sulfides, respectively.
 On Thursday 30th September 2021, Marek Tuhý successfully defended his PhD thesis entitled “Wildfires in polluted areas: mineralogical transformations and remobilization of metal(loid)s“. The thesis was supervised by prof. Vojtěch Ettler and reviewed by Dr. Isabel Campos (University of Aveiro, Portugal) and Dr. Tomáš Navrátil (Institute of Geology, CAS). Congratulations!
On Thursday 30th September 2021, Marek Tuhý successfully defended his PhD thesis entitled “Wildfires in polluted areas: mineralogical transformations and remobilization of metal(loid)s“. The thesis was supervised by prof. Vojtěch Ettler and reviewed by Dr. Isabel Campos (University of Aveiro, Portugal) and Dr. Tomáš Navrátil (Institute of Geology, CAS). Congratulations! A new book entitled Metallurgical Slags: Environmental Geochemistry and Resource Potential edited by Nadine M. Piatak (USGS) and Vojtěch Ettler has been published by the prestigious publishing house, the Royal Society of Chemistry (
A new book entitled Metallurgical Slags: Environmental Geochemistry and Resource Potential edited by Nadine M. Piatak (USGS) and Vojtěch Ettler has been published by the prestigious publishing house, the Royal Society of Chemistry (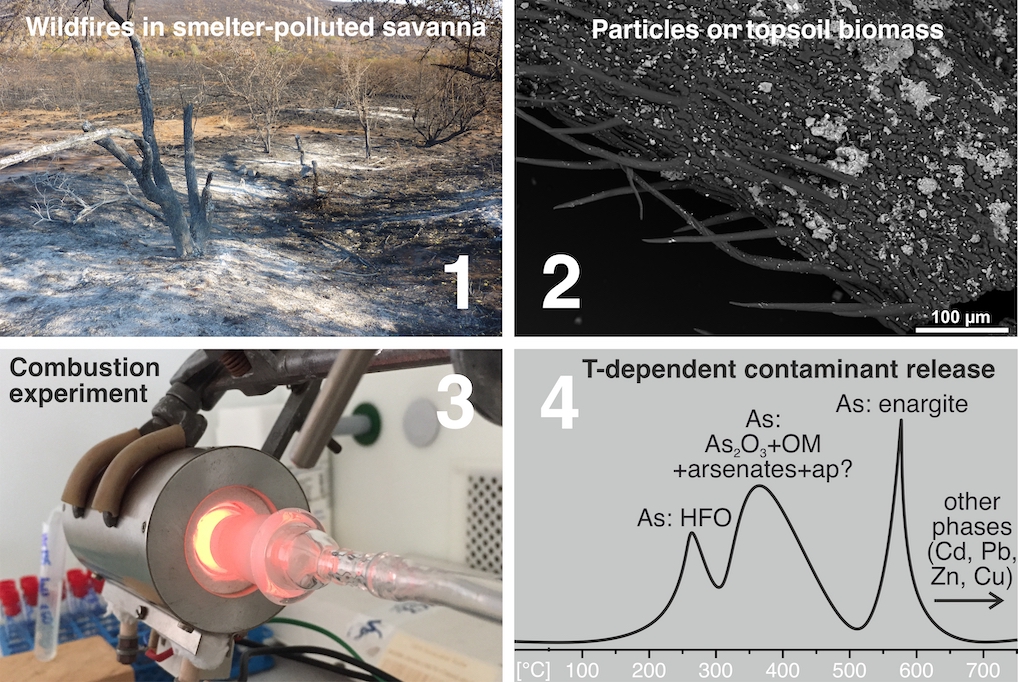 The temperature-dependent releases of metal(loid)s (As, Cd, Cu, Pb, Zn) from biomass-rich savanna soils collected near a Cu smelter in Namibia have been studied under simulated wildfire conditions. For this purpose, new wildfire-simulating setups were introduced. Laboratory single-step combustion experiments (250–850 °C) and experiments with a continuous temperature increase and online ICP-OES detection (25–750 °C) were coupled with mineralogical investigations of the soils, ashes, and aerosols. The results indicate that metals are dominantly concentrating in the ash residue, and part of As is remobilized depending on temperature. Therefore, the active and abandoned mining and smelting sites, especially those highly enriched in As, should be protected against wildfires, which can be responsible for substantial As re-emissions.
The temperature-dependent releases of metal(loid)s (As, Cd, Cu, Pb, Zn) from biomass-rich savanna soils collected near a Cu smelter in Namibia have been studied under simulated wildfire conditions. For this purpose, new wildfire-simulating setups were introduced. Laboratory single-step combustion experiments (250–850 °C) and experiments with a continuous temperature increase and online ICP-OES detection (25–750 °C) were coupled with mineralogical investigations of the soils, ashes, and aerosols. The results indicate that metals are dominantly concentrating in the ash residue, and part of As is remobilized depending on temperature. Therefore, the active and abandoned mining and smelting sites, especially those highly enriched in As, should be protected against wildfires, which can be responsible for substantial As re-emissions. The paper presents a geochemical analysis of a remarkable assemblage from the early La Tène period (4th century BCE): the Duchcov hoard found in the late 19th century in north-western Bohemia. More than a thousand pieces of bronze jewellery in a bronze cauldron were deposited in a natural spring. This possibly ritual offering of unknown purpose might have involved a large community whose origin and structure could be discussed using archaeometric data from the hoard. This assemblage offers a unique opportunity to study Iron Age bronze metalworking. The results show that this seemingly homogeneous assemblage contains several chemically distinctive groups that are compatible with the spread of the so-called Duchcov-Münsingen horizon in the 4th century BCE.
The paper presents a geochemical analysis of a remarkable assemblage from the early La Tène period (4th century BCE): the Duchcov hoard found in the late 19th century in north-western Bohemia. More than a thousand pieces of bronze jewellery in a bronze cauldron were deposited in a natural spring. This possibly ritual offering of unknown purpose might have involved a large community whose origin and structure could be discussed using archaeometric data from the hoard. This assemblage offers a unique opportunity to study Iron Age bronze metalworking. The results show that this seemingly homogeneous assemblage contains several chemically distinctive groups that are compatible with the spread of the so-called Duchcov-Münsingen horizon in the 4th century BCE.